Now there’s a proactive tool to screen for cancer
The Galleri test can be taken annually as a simple blood test and screens for a “fingerprint” of many of the deadliest cancers before they become symptomatic, including those with no recommended screening tests today.
The Galleri test does not detect a signal for all cancers and not all cancers can be detected in the blood. False positive and false negative results do occur. The Galleri test should be used in addition to healthcare provider recommended screening tests.
Who is the Galleri test for?
The Galleri test is recommended for adults with an elevated risk for cancer, such as those age 50 or older.
More than 1 in 3 people will develop cancer in their lifetime.6 People over the age of 50 have a 13 times higher risk for cancer than those under 50. Cancer risk increases for everyone as they age, regardless of family history7 — only 5% to 10% of cancers are inherited.7,8
The Galleri test is recommended for use in adults with an elevated risk for cancer, such as those aged 50 or older.
Talk to your provider about your risk for cancer, and whether the Galleri test is right for you.
- The Galleri test is available by prescription only.
- Use of the Galleri test is not recommended in individuals who are pregnant, 21 years old or younger, or undergoing active cancer treatment.
Age is the biggest risk factor for cancer. Adults over age 50 are 13 times more likely to have cancer compared to people under the age of 50.
Certain factors, in addition to age 50+, further increase cancer risk5 :
- Diabetes
- Obesity (BMI ≥ 30)
- Current or previous smokers

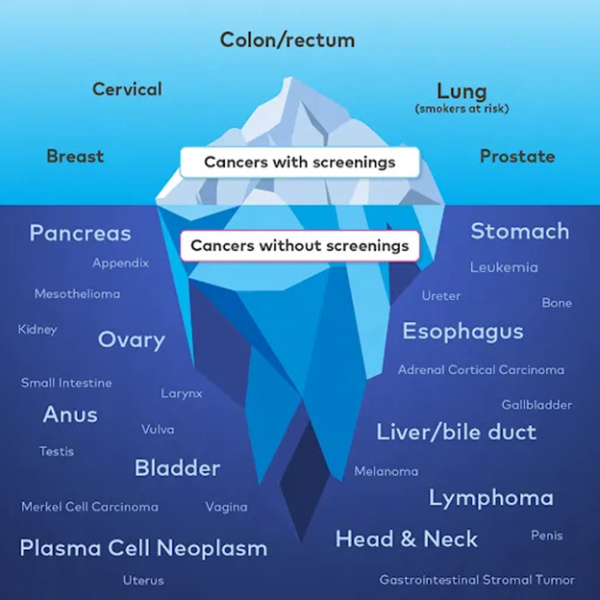
How Does the Galleri Test look for Cancers?
Cancers growing in the body shed DNA into the bloodstream.1,2,3 Although there are many types of cancer, the DNA fragments can act like a unique “fingerprint” of cancer. This Galleri test screens for many of the deadliest cancers before they become symptomatic, including those without recommended screening tests.1,4 When there is a Cancer Signal Detected, the results also provide predicted Cancer Signal Origin* to help your healthcare provider determine the next steps for diagnosis.
While single-cancer screening tests play an important role in detecting 5 specific cancers today, we can do more. When cancer is diagnosed early, before it has time to spread, the overall 5-year cancer-specific survival rate is 4 times higher than when cancer is diagnosed late.
Laboratory & Test Information :
The GRAIL clinical laboratory is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and accredited by the College of American Pathologists. The Galleri test was developed — and its performance characteristics were determined — by GRAIL. The Galleri test has not been cleared or approved by the Food and Drug Administration. The GRAIL clinical laboratory is regulated under CLIA to perform high-complexity testing. The Galleri test is intended for clinical purposes.



